Gummy Bear Osmosis Experiment
Gummy Bear Osmosis experiment is a fun demonstration to help explain the tricky subject of osmosis, as well as being a great way to teach experimental design. It’s also an experiment you can eat when you’re finished! Read on to find out why the Gummy Bear Osmosis Experiment is so good at teaching osmosis (obviously!), but also experimental design, mass, weight & volume as well as embedding maths.
OK, so lets start at the beginning……
What is Osmosis?
Osmosis is the diffusion of water molecules from a dilute liquid (where there are many water molecules) to a more concentrated solution (where there are fewer water molecules) across a selectively permeable membrane. Selectively permeable is a membrane which allows some molecules to pass through but inhibits others. These membranes can be found in nature, such as membranes surrounding cells or synthetically made membranes.
Why a Gummy Bear?
The Gummy Bear has a selectively permeable coating which will allow water molecules to diffuse across, but inhibiting other larger molecules. In this osmosis experiment the water molecules move into the bear, thus enlarging it.
Material used
You’ll need these things:
- Gummy bears
- Cups
- Water (and other liquids for the extended project)
- Paper towels
- Kitchen item for measuring liquids, e.g. measuring cup/jug/cylinder.
- Weighing scales/balance that can weigh to 0.1g increments.
Method
1) Take 2 gummy bears that are the same colour and similar size. Record their weights*.
*see Lab Notes below.

Record the weight of the gummy bear before the experiment
2) Take 2 identical cups, and put a gummy bear in each. Fill ONE cup with water so that the bear is submerged, but measure the amount of water you use as this information will be useful later on. The other cup remains dry (no water). This is your control bear which will prove the bear does not enlarge without water!

Set up of the TEST gummy bear.

Set up a control gummy bear (no water)
3) Leave both bears for several hours, or overnight preferably and then compare gummy bears.

Gummy Bear Osmosis – the results
4) The next day, take the bears out of their cups, trying not to lose any water from the wet bear, and pat the wet one dry with a paper towel.
Now weigh both bears, and record the results.
Calculate the weight gained by the enlarged bear by subtracting the weight before from the weight after.
We need to check the weight of the control bear as well, as this may have changed too.
Take this a step further if you want to, by calculating the percentage weight* gained.
* see Lab Notes below.

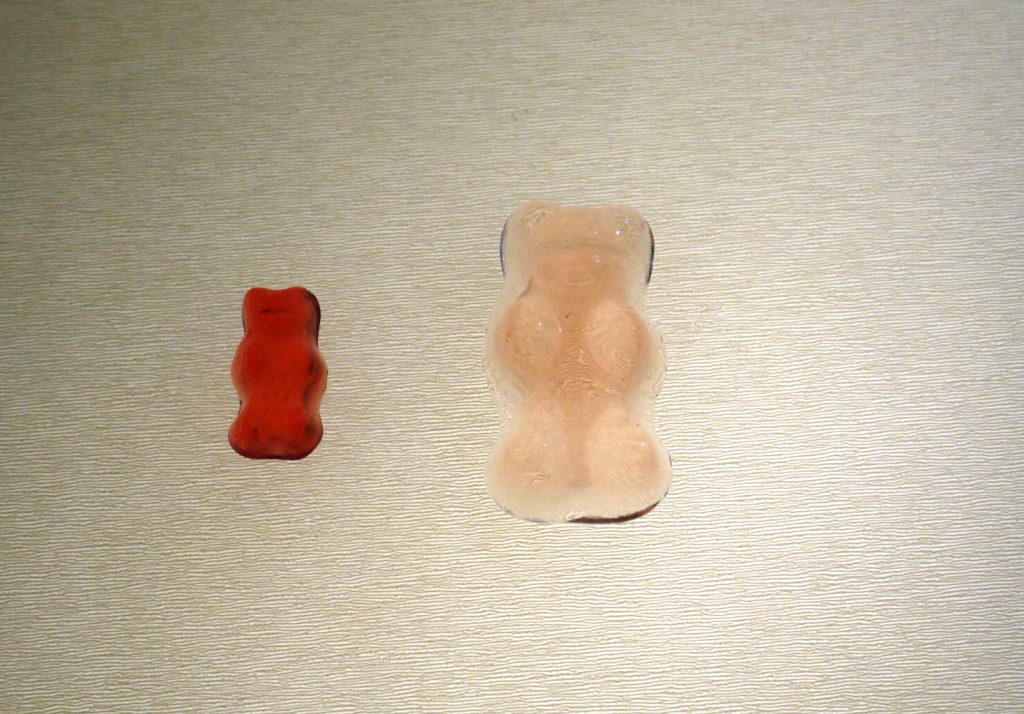
Comparison of before & after weights – this Gummy Bear gained 6.39g
5) Finally, measure the volume of water left behind in the enlarged bear’s cup…has it altered? Where has it gone??
How did the bear get so BIG?
Well, we already know from the introduction that water diffuses from an area of high concentration of water molecules (i.e. a dilute solution) to an area of low water concentration. In this experiment water moves into the Gummy Bear, where there are fewer water molecules, making it swell up. The water keeps moving until the water molecules are evenly spaced out (i.e. have reached an equilibrium). The molecules stop diffusing when they reach equilibrium.
Make this a bigger project.
So we put our bear in water, which obviously has lots of water molecules in, but what if our liquid had fewer water molecules compared to the bear. What would happen? Which direction would the water molecules move and what effect would that have in the bear?
……Over to you to find out….
Extend this experiment by trying different liquids, such as vinegar, salty water, juice and so on. Make sure you set them all up at the same time so the bears spend an equal amount of time in each liquid, and always include a control Gummy Bear (bear in no liquid).
Lab notes
Why must the bear be the the same colour?
Changing the colour adds a new variable to the experiment, and you need to be sure that any change in the gummy bear size is due to the water you are putting them in, and not their colour!
Why do they have to be the same size?
Well they don’t really have to be, but they should be about the same size, for the same reasons we need the colour to be the same. You are calculating the change in weight, which is more accurate than just looking at the bears and saying that one is bigger than the other! What does “bigger” mean?
How to calculate percentage weight gain.
To find out the percentage gain, you divide the weight gained by the start weight and multiply by 100.
This is useful because it allows you to compare the results of different Gummy Bear osmosis experiments, where the start weight will be slightly different for each bear.
Lets look at my example above:
The bear gained was 6.39 grams and the start weight was 1.7g….so, 6.39g divided by 1.7g = 3.76g x 100 = 376%. So the bear has an increase in weight of 376%.
If you tackle this activity at home you do so at your own risk. If you have as much fun as we did, feel free to share your pictures with us on our Facebook page.
Ruth
Chief Scientist at Devon Science
Want more easy science to do?
So you liked this activity and want more ideas, right? Well head on over to our blog to find out other cool experiments such as Glowing Fluorescent Liquid or the Super Easy 6 – a free download with 6 super-easy experiments to try at home!